Paragard IUD Lawsuits
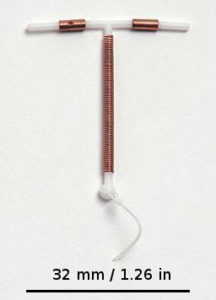
Paragard is a flexible plastic intrauterine device (IUD) used for long-term birth control. It is the only non-hormonal IUD available in the United States. Paragard was approved by the FDA in 1984. Also called a copper IUD, the T-shaped device is wrapped in copper coils. The copper induces an inflammatory response in the uterus, which creates a spermicidal effect.

Home » Defective Medical Device Lawyer » Paragard IUD Lawsuit
The device is approved for implantation for up to 10 years. The doctor removes the IUD by gently pulling two strings that are attached to the IUD, which remain just outside the cervix while the IUD is present.
Unfortunately, a growing number of women have experienced severe complications after using the device, many of which have resulted in permanent infertility, pain, invasive surgeries, and severe emotional distress.
Manufacturers have a duty to provide safe products, and when their products cause injuries, they must be held accountable, especially when those products are harmful medical devices. The makers of Paragard are no exception. The dedicated attorneys at Milavetz Injury Law, P.A., are committed to holding the manufacturers of Paragard accountable and ensuring victims receive just compensation.
What are the claims against manufacturers?
Multiple lawyers are now investigating the design, marketing, and promotion of the flawed devices leading many to take legal action against the company. The most common complaint that has triggered Paragard lawsuits is breakage of the device during removal, resulting in fragments remaining inside the uterus. This often necessitates surgery to remove the pieces.
The plastic on the Paragard IUD is intended to be flexible, so the top arms of the “T” should fold down to facilitate intact removal. Sometimes, one or both arms of the T break off, leaving one or more fragments inside the uterus.
If not removed, IUD fragments can cause serious complications that could be life-threatening. Removal is not always simple, and some women have been forced to undergo complete hysterectomies, resulting in permanent infertility and severe emotional distress.
Paragard was manufactured by Teva Pharmaceuticals until CooperSurgical purchased the brand. The issues were already known prior to this sale, but CooperSurgical continued manufacturing the same product.
Breakage during removal is not the only complication women have experienced. Some have reported complications during insertion and throughout the duration of their use of Paragard. These complications along with breakage during removal have resulted in serious injury and illness, including the following:
- The necessity of invasive procedures to remove the IUD
- Hysterectomy – removal of the uterus
- Laparotomy – an invasive surgery similar to a caesarian section
- Laparoscopy – similar to laparotomy but with a smaller incision to find missing fragments
- Hysteroscopy – a less invasive procedure that requires general anesthesia
- Pelvic Infections
- Inflammation
- IUD embedding in the uterus or other organs
- IUD migration to other organs
- Permanent loss of fertility
- Pain
- Perforation of the uterus or other organs
- Sepsis
Manufacturers owe a duty of care to create safe products. Negligence occurs when manufacturers breach that duty. Teva Pharmaceuticals and CooperSurgical breached their duty of care when they continued to offer dangerous products to the public after safety issues were reported. They have not modified the product, and they are refusing to take responsibility for the injuries their products have caused thousands of unsuspecting women.
The lawsuits against Teva Pharmaceuticals and CooperSurgical allege that they have breached their duty of care on the following grounds:
- Defective design – Teva Pharmaceuticals and CooperSurgical have manufactured a poorly designed product that is prone to breakage.
- Manufacturing defects – Rather than fault the design, some lawsuits allege the manufacturers allow defective products to reach the public, which would indicate quality control deficiencies.
- Failure to warn – The defendants have failed to advise physicians and the public about the potential for breakage and how to mitigate these risks.
Teva Pharmaceuticals and CooperSurgical are facing a growing number of lawsuits as a result of their faulty products, which have seriously injured women who trusted Paragard as a safe and effective method of birth control.
In addition, the FDA has received over 41,000 reports of complications arising from Paragard, including 17,208 complaints of injuries that were severe or resulted in death. This is clear and convincing evidence that Teva Pharmaceuticals and CooperSurgical have knowingly and negligently manufactured dangerous products.
Status of Paragard Lawsuits
On December 16, 2020, a large number of Paragard cases were consolidated and transferred to the United States District Court in the Northern District of Georgia on docket 2974. The consolidation of cases is called multidistrict litigation, or MDL.
MDL is used when a large number of similar cases are tried in the federal courts. The purpose of MDL is to increase the efficiency of the process and prevent congestion in the courts by having one judge manage all the cases and in some cases make a single ruling about the merits of the cases.
MDL cases differ from class-action lawsuits. In class-action lawsuits, the cases are combined into one case and litigants split a single award. In MDL, the cases remain separate, and each litigant’s award is still individually decided.
MDL offers the following benefits to plaintiffs:
- The process moves more quickly
- The filing process is simplified
- The cost of litigation is lower
As of January 2022, 797 plaintiffs have joined. New litigants may file short-form complaints into the MDL, so long as their cases meet the following eligibility requirements:
- The case alleges injuries were caused by breakage of the Paragard IUD during removal.
- The defendants are Teva Pharmaceutical and CooperSurgical.
There have been no settlements or judgments in Paragard cases yet. Typically, when cases are transferred to MDL, a few of the cases will go to trial. These are called bellwether trials. The outcomes of bellwether trials are used as guidance to determine compensation in future settlements and lawsuits. Bellwether trials can have a significant impact on pending cases. For example, if the plaintiffs prevail, the defendants will be more willing to offer reasonable settlements.
Should you file a Paragard lawsuit?
Although there have not been any settlements yet, we anticipate compensation will be substantial when these cases do start to settle. This is due to the number of victims, the severity of their injuries, and the failure by the manufacturers to make their products safe even after they knew about the complications.
It is important to act now. The court battle against the manufacturers of Paragard is underway, and the sooner we start your case, the better your chance of receiving compensation when the consolidated cases are heard by the court.
At Milavetz Law, we can evaluate your case and determine whether it is eligible for the MDL docket. If so, we can file your case into the MDL. If not, we will file an individual action on your behalf. Either way, we are committed to holding these manufacturers accountable.
If you have used Paragard®, during the years 2005–present, and have suffered breakage of this device during removal, please contact us to see how we can help.
Discuss Your Legal Questions With A Member Of Our Team
"*" indicates required fields




Speak to an Attorney Today
Let Us Help You With Your Financial Help & Compensation Options